Time : Marzo 8, 2024
Abstract: The principle of electric polishing for a stainless steel cage is introduced, and the polishing process conditions and factors affecting polishing quality are analyzed. Actual use shows that this process can meet production needs, and the polished stainless steel cage obtains a uniform bright layer.
Keywords: Rolling bearings; Retainer; Stainless steel; Electric polishing
The process of putting a workpiece into a specific solution for anodic electrolysis to make the metal surface smooth and produce a metallic luster is called electrolytic polishing or electro polishing. It removes the surface layer generated during the mechanical polishing process and generates a metal oxide layer with high corrosion resistance and reflectivity on the surface, while also reducing surface stress and friction coefficient. Electrolytic polishing is the anodic electrochemical corrosion process on the surface of stainless steel. When using stainless steel workpieces as the anode for electrolysis, the first step is the electrochemical and chemical dissolution of the surface oxide layer and metal, as well as the physical erosion of oxygen bubbles that precipitate on the workpiece, causing the surface layer to detach from the surface of the workpiece and be removed.
Electrochemical polishing may undergo the following reactions:
(1) Metal atoms lose electrons and transform into metal ions entering the solution
M → M2++2e
(2) Formation of oxide film
M+H2O → MO+2H++2e
(3) Oxygen precipitation
2H2O → O2+4H++4e
The process of electrolytic polishing can be explained by mucosal theory as follows: when using stainless steel workpieces as anodes for electrolytic polishing, if the dissolution rate of the anode is greater than the diffusion rate of the anodic dissolution products from the anode surface to the depth of the electrolyte, the dissolution products will accumulate near the anode surface. A viscous liquid film with high resistance is formed, and its distribution on the anode surface is uneven. The mucosa at the protrusion is thin and has low electrical resistance. High current density, high oxygen precipitation, easy solution renewal, and fast dissolution rate; The concave mucosa is thick, with high resistance, low current density, and slow dissolution. As the polishing time continues, the protrusions on the anode surface are gradually flattened, making the surface smooth and smooth.
2.1 Pre polishing treatment
Austenitic stainless steel and martensitic stainless steel are commonly used materials for stainless steel cages. Most austenitic stainless steel cages have relatively clean surfaces and can be polished directly without pre-treatment as needed. Martensitic stainless steel cages require heat treatment such as quenching and tempering, and their surface has a thick layer of oxide skin and oil stains. If not removed, it will seriously affect the polishing quality and can be removed in a pre-treatment solution. The pre-treatment solution formula is based on volume fraction: H2SO4 20% -25%, HCI 5% -8%, HNO3 3% -7%, glycerol 1% -2%, and residual distilled water.
H2SO4, HCI, and HNO3 in the above pre-treatment solution can remove the oxide scale and oil stains on the surface of the cage. Glycerol can prevent corrosion. Soak the workpiece in this solution for 3-8 minutes and stir appropriately until the oxide scale is removed. After cleaning with flowing water, remove the water and set it aside for use.
2.2 Electrolytic polishing
The polishing solution formula is based on a mass fraction of H3PO4 50% -60%, H2SO4 10% -18%, CrO3 4% -8%, glycerol 14% -18%, and distilled water residue.
The appearance of the front and rear axle bearing brackets before polishing is shown in Figure 1: the middle black represents before polishing, and the surrounding light represents after polishing.
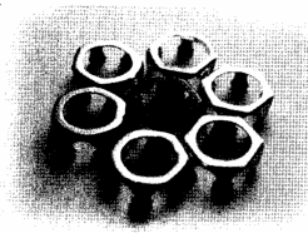
Figure 1 Polishing the appearance of the front and rear holders
3.1 Effects of electrolyte composition
(1) Phosphoric acid. During the electrolysis process, it can promote the formation of a barrier film on the polished surface, which has a certain inhibitory effect on the dissolution of stainless steel, thereby improving anode polarization and achieving mirror gloss on the polished surface. When the content is high, the resistance of the tank liquid increases, and the viscosity increases, resulting in a higher required voltage, which slows down the leveling speed; The content is too low and the activation tendency is small, resulting in uneven corrosion on the surface of the workpiece.
![]()
When its content is too low, the surface of the polished workpiece is not bright; When the content is too high, it will reduce the polishing speed.
(4) Glycerol. Glycerol can adsorb onto the surface of the anode and have a certain inhibitory effect on anodic dissolution. It can also form a C3H5 (OH) 2PO4 complex with phosphoric acid, which can form a stronger blocking film on the anode surface, preventing the dissolution of the anode and making the polished surface very bright and fine. When its content is too low, the polished surface is bright but rough; When the content is high, the polished surface is bright and fine, but when the content is too high, too much foam affects the operation.
3.2 Impact of operating conditions
3.2.1 Current density
In most cases, the anode current density is almost proportional to the amount of dissolved metal. Only by selecting a good anode current density and controlling it within a certain anode potential range can good polishing quality be achieved. There is a suitable current density for any metal electro polishing system. Generally speaking, if the current density is too low and the electrode is in an activated state, the surface will undergo etching due to the anodic dissolution of the metal, resulting in a rough surface; When the current density is too high, a large amount of oxygen will precipitate, covering the local surface of the anode and causing poor conductivity. It may also cause local overheating of the anode surface, resulting in excessive corrosion of the metal surface.
3.2.2 Tank liquid temperature
The temperature of the polishing solution should be maintained within the specified process range to ensure the normal polishing and leveling speed of the stainless steel surface, in order to effectively reduce the viscosity of the electrolyte, reduce the thickness of the anode mucosa, accelerate the diffusion of anodic dissolution products, accelerate solution convection, facilitate the detachment of trapped bubbles on the anode, and avoid the formation of spots and pitting. If the temperature is too high, it can cause the solution to overheat, accelerate the transition from hexavalent chromium to trivalent chromium (Cr6++3e → Cr3+), and easily cause surface corrosion. The generated gas and vapor may push the polishing solution away from the metal surface, thereby reducing the polishing effect. If the temperature is too low, the viscosity of the solution will increase, and the surface mucosa of the anode will thicken, which is not conducive to the diffusion of anodic dissolution products and significantly reduces the polishing and leveling effect.
3.2.3 Polishing time
The polishing time depends on the following factors: the original surface state of the metal product, the current density and temperature used, the composition of the electrolyte, and the properties of the metal. During the initial period of electro polishing, the leveling speed Z of the anode surface is faster, and then it becomes slower and even increases in surface roughness after a certain period of time. Therefore, the polishing time should take into account the above factors comprehensively. In general, as the current density increases and the temperature increases, the polishing time should be shortened. When the original surface quality of the workpiece is good and the requirements are high, the polishing time should be shortened.
3.2.4 Anode movement
Anode movement accelerates the diffusion of anodic dissolution products, playing a stirring role, effectively eliminating bubbles trapped on the anode surface, avoiding the generation of streaks in the generated airflow, and preventing local overheating from causing surface corrosion; And it can update the polishing solution near the anode surface, making the temperature of the polishing solution more uniform, preventing local overheating of the metal surface, and accelerating the dissolution rate of the mucosa; Anode movement helps to increase anode current density and improve the electrochemical polishing quality of stainless steel parts.
3.2.5 Cathode materials
The cathode for electrolytic polishing generally chooses lead plate. From the perspective of current efficiency, increasing the cathode area is beneficial, but increasing the cathode area will accelerate the reduction rate of Cr6+to Cr3+. The cathode area should be controlled within 1/2-1/3.5 of the anode area to prevent the growth of trivalent chromium. Excessive trivalent chromium content can easily cause the polishing solution to age.
(1) Stainless steel workpieces must be thoroughly degreased before polishing to prevent oil contamination from contaminating the polishing solution.
Through practical verification, the introduced process has a good polishing effect on stainless steel cages, with a bright and uniform surface, which can meet the quality requirements of electric polishing for stainless steel bearing cages. Especially for heat-treated martensitic stainless steel cages, it can effectively solve the problems of uneven polishing and localized corrosion, and improve the surface smoothness and corrosion resistance of the workpiece.
2024 March 2nd Week XZBRG Product Recommendation:
Flanged ball bearings have a solid steel flange on the outer ring. This allows the bearing to be more easily located in a housing. The flange can also help to prevent axial movement of the bearing in the event of a thrust load on the bearing. It is easier to maintain the position of a flanged bearing in the housing where a lot of vibration is present.
http://xinzhou.bearingshow.net/product-cat/flanged-ball-bearings/
